Article Contents
Title: Unraveling the Marvels of Endothermic Reactions: Exploring Chemistry’s Cold Comfort
Introduction to endothermic reaction
In the vast realm of chemistry, one encounters a myriad of fascinating phenomena, from explosive reactions to colorful transformations. Among these captivating occurrences, endothermic reactions stand out as remarkable examples of energy absorption. In this article, we delve into the world of endothermic reactions, uncovering their distinctive characteristics, real-world applications, and their significance in various scientific fields. So, let’s embark on a chilly journey to understand the captivating nature of these cold comfort reactions.

Understanding Endothermic Reactions
Endothermic types of reactions are chemical processes that absorb energy from their surroundings, typically in the form of heat. As a result, these reactions feel cold to the touch, hence the prefix “endo” meaning “within” in Greek. To comprehend the essence of endothermic reactions, it is crucial to grasp the concept of energy exchange in chemical systems.

Every chemical reaction involves a breaking and formation of chemical bonds, which requires the input or release of energy. Endothermic reactions have a higher energy requirement for bond breaking than for bond formation, resulting in an overall energy absorption. This deficit in energy is compensated by drawing heat energy from the surroundings, causing a drop in temperature.
Key Characteristics of Endothermic Reactions
- Heat Absorption: The primary characteristic of endothermic types of reactions is their capacity to absorb heat energy. This property distinguishes them from exothermic reactions, which release heat energy into the environment.
- Temperature Decrease: As endothermic reactions absorb heat, they cause a noticeable drop in temperature within the system or its surroundings. This decrease can be observed through touch or by using a thermometer.
- Positive Energy Change: Endothermic type of reactions exhibit a positive value for their enthalpy change (∆H), indicating that energy is being consumed during the reaction.
Real-World Applications:
- Cold Packs: Endothermic kind of reactions find practical use in the creation of instant cold packs. These packs typically consist of two compartments that, when mixed, initiate an endothermic reaction. The absorption of heat cools the pack, making it suitable for treating minor injuries and reducing swelling.
- Cooling Systems: Endothermic reactions are utilized in cooling systems such as air conditioners and refrigerators. These devices employ chemical compounds with endothermic properties to absorb heat from the surrounding environment and lower the temperature.
- Self-Cooling Beverages: In the food and beverage industry, endothermic reactions play a role in self-cooling beverage containers. When a button is pressed or a seal is broken, a chemical reaction occurs, absorbing heat and rapidly cooling the beverage inside the container.
Scientific Significance:
Endothermic kind of types of reactions hold great scientific importance across various fields:
- Thermodynamics: The study of endothermic types of reactions contributes to the understanding of energy changes within chemical systems, which is fundamental to the field of thermodynamics.
- Kinetics: Investigating the rate at which endothermic reactions occur provides insights into reaction mechanisms and allows scientists to fine-tune reaction conditions for improved efficiency.
- Material Science: Endothermic type of reactions have implications in material science and engineering. They are crucial in processes like solid-state cooling, where materials with endothermic properties are used to create cooling effects in electronic devices.
Conclusion:
Endothermic reactions are captivating phenomena in the realm of chemistry, showcasing the ability of certain chemical systems to absorb heat energy from their surroundings. Their distinct characteristics, such as temperature decrease and positive energy change, set them apart from other types of reactions. Understanding and harnessing the power of endothermic reactions has led to a range of practical applications, including cold packs, cooling systems, and self-cooling beverages.
Read Also,
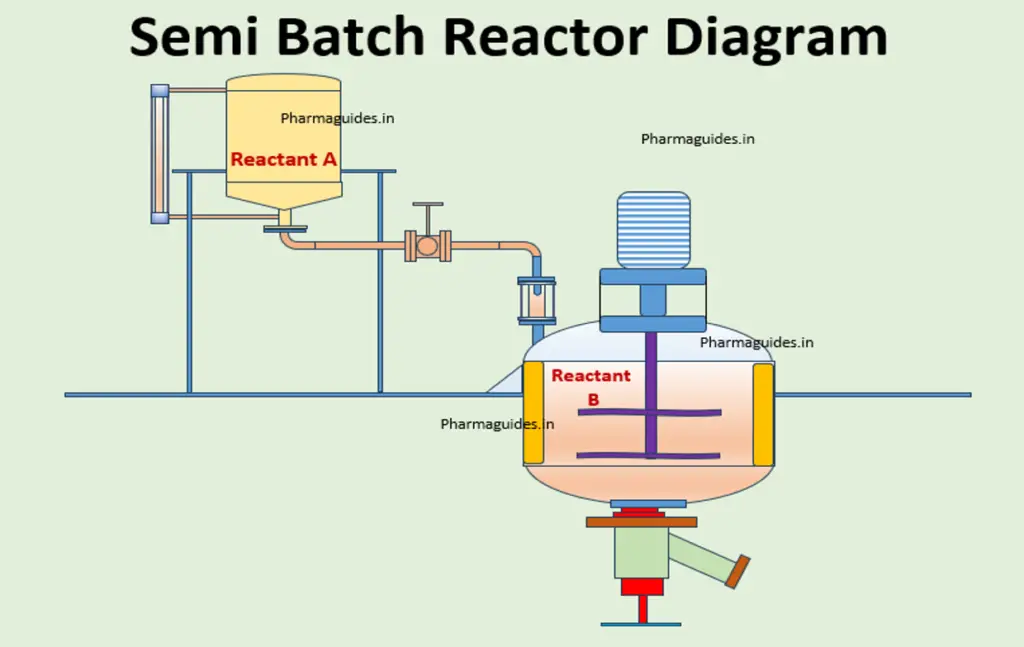
what are the different types of reactor used in chemical industry
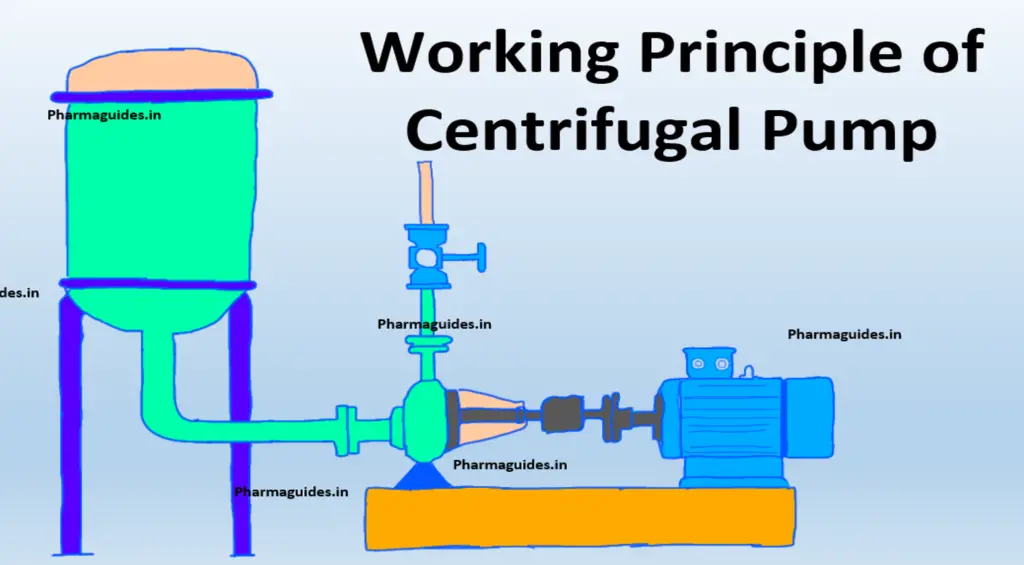
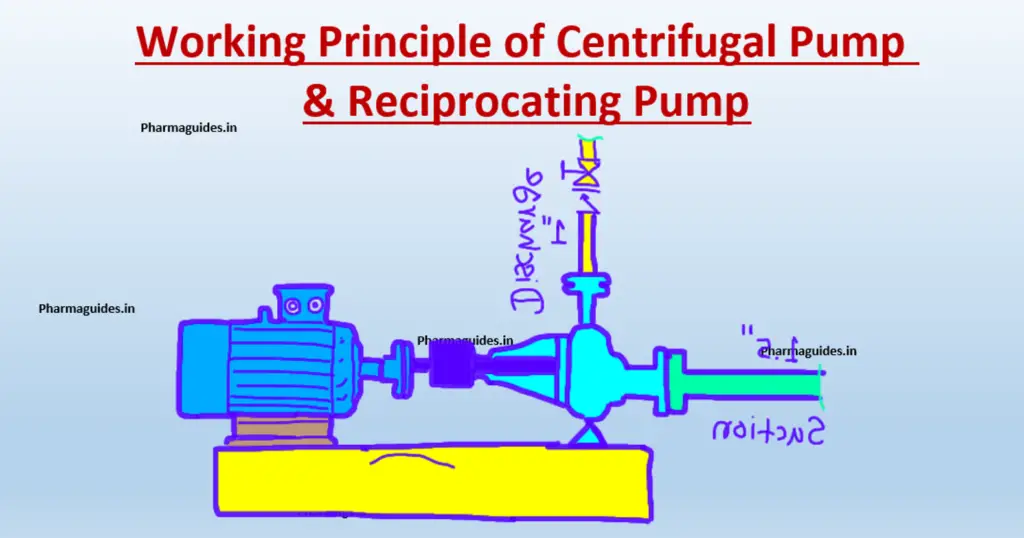
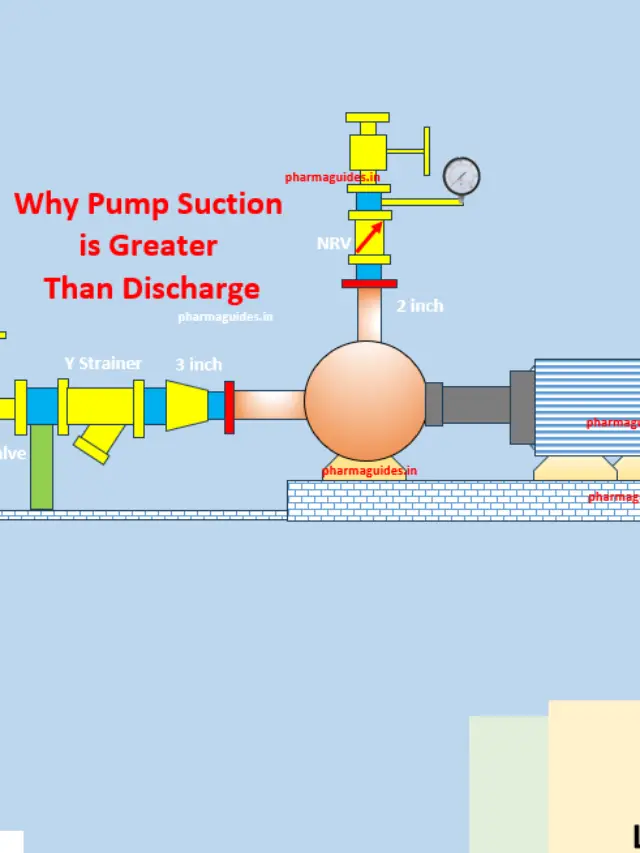
difference between Piston pump and gear pump
types of stirrer use in reactor





1 COMMENTS
Comments are closed.