Article Contents
Distillation: A Separation Process
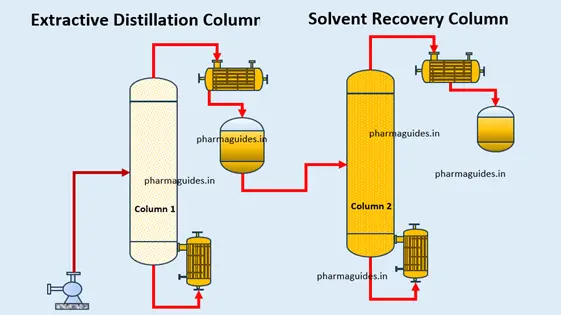
Extractive distillation :- Distillation, a stalwart among separation processes, is a technique widely employed to purify substances based on differences in boiling points. In the expansive field of distillation techniques, extractive distillation stands out as a versatile and nuanced approach to separation challenges, offering a unique twist to the traditional distillation process.

What is Extractive Distillation?
Extractive distillation represents a departure from conventional distillation by introducing a solvent into the process. This solvent plays a pivotal role in modifying the relative volatility of components within the mixture. Unlike fractional vaporization alone, extractive kind of distillation exploits the selective interaction between the solvent and mixture components to achieve a more refined separation.
Destructive Distillation Process
What is Destructive Distillation
Destructive Distillation of wood
Destructive Distillation of Coal
Why Extractive Distillation?
Key points explaining why extractive distillation is important:
- Overcoming Limitations of Traditional Distillation:
- Extractive distillation addresses the limitations of traditional distillation methods, especially when dealing with mixtures that have components with similar boiling points or form azeotropic mixtures.
- Precision in Separation:
- The use of a solvent in extractive distillation allows for a more precise separation of components, providing a nuanced approach to tackle complex mixtures and achieve higher purity levels in the final products.
- Efficient Handling of Azeotropic Mixtures:
- Extractive distillation is particularly crucial when facing azeotropic mixtures, where traditional distillation struggles to completely separate components. The introduction of a solvent helps break these azeotropes and facilitates effective separation.
- Strategic Solvent Utilization:
- The strategic use of solvents enables customization of the separation process. Solvents can be tailored to interact selectively with specific components, enhancing the overall efficiency of the distillation.
- Versatility Across Industries:
- The adaptability of extractive kind of distillation makes it a valuable technique across diverse industries such as petrochemicals, pharmaceuticals, environmental processes, and more. Its versatility allows it to address varied separation challenges in different industrial settings.
- Improved Product Quality:
- Extractive kind of distillation often results in higher-purity products, meeting the stringent quality requirements of industries such as pharmaceuticals and specialty chemicals. This is crucial for ensuring the reliability and effectiveness of the final products.
- Recycling Valuable Components:
- The method enables the recycling of valuable components from complex mixtures, contributing to sustainable and resource-efficient processes. This is particularly relevant in industries striving for eco-friendly and economically viable practices.
- Strategic Application in Renewable Energy:
- In the context of renewable energy, extractive distillation plays a strategic role in improving the separation of components in biofuel production, contributing to the efficiency and viability of sustainable energy sources.
- Meeting Industry-Specific Challenges:
- Extractive kind of distillation is important because it provides solutions to industry-specific challenges related to separation, ensuring that processes can be tailored to meet the unique demands of each sector.
- Continuous Advancements in Process Efficiency:
- Ongoing research and advancements in extractive distillation techniques contribute to increased process efficiency, making it an important area for innovation and optimization in separation processes.
Extractive Distillation Nitric Acid
Extractive types of distillation can be employed for the separation of nitric acid from other components in a mixture. Nitric acid is a highly corrosive and reactive chemical, and its separation often involves challenges due to its tendency to form azeotropes with water. Extractive kind of distillation can be a valuable technique in this context. Here’s an overview of extractive type of distillation applied to nitric acid separation:

- Challenges in Nitric Acid Separation:
- Nitric acid commonly forms azeotropic mixtures with water, making traditional distillation less effective. Azeotropes are challenging to break using conventional methods.
- Introduction of Extractive Distillation:
- Extractive distillation involves the addition of a solvent to the mixture to modify the relative volatility of components. In the case of nitric acid separation, a suitable solvent is introduced to facilitate the extraction of nitric acid from the mixture.
- Solvent Selection:
- The choice of solvent is critical. Common solvents for nitric acid extraction include organic compounds like tributyl phosphate (TBP) or tri-n-butylamine. The solvent should have a distinct boiling point from nitric acid and water, allowing for efficient separation.
- Boiling Point Consideration:
- The solvent’s boiling point should be carefully selected to ensure it is lower than that of nitric acid and higher than that of water. This ensures that the solvent can be easily separated from the mixture after extraction.
- Process Overview:
- Nitric acid and the chosen solvent are mixed, and the mixture is subjected to distillation. The solvent selectively interacts with nitric acid, breaking the azeotrope and facilitating the separation of nitric acid from water.
- Separation Efficiency:
- Extractive type of distillation enhances the separation efficiency by modifying the vapor-liquid equilibrium in favor of nitric acid. This results in a higher concentration of nitric acid in the distillate, improving the overall separation process.
- Recovery of Solvent:
- After distillation, the solvent can be recovered for reuse in subsequent cycles. This contributes to the sustainability of the process and reduces the overall operational costs.
- Application in Nitric Acid Production:
- Extractive distillation is often applied in nitric acid production processes where high-purity nitric acid is required. The method enables the production of nitric acid with reduced water content, meeting the specifications for various industrial applications.
- Control of Corrosion and Reactivity:
- The use of extractive distillation with suitable solvents can help control the corrosive and reactive nature of nitric acid, providing a safer and more controlled separation process.
- Advantages:
- Extractive distillation offers advantages such as improved separation efficiency, the ability to break azeotropes, and the production of higher-purity nitric acid compared to traditional distillation methods.
When Should You Use Extractive Distillation?
Extractive distillation is a specialized separation technique, and its application is particularly beneficial in specific scenarios where conventional distillation methods may encounter limitations. Here are situations in which you should consider using extractive distillation:
- Azeotropic Mixtures:
- Scenario: When dealing with azeotropic mixtures where traditional distillation struggles to break the azeotrope and achieve complete separation.
- Reason: Extractive distillation can effectively break azeotropes by introducing a solvent that alters the vapor-liquid equilibrium, facilitating the separation of components.
- Components with Similar Boiling Points:
- Scenario: When the components in the mixture have similar boiling points, making it challenging to achieve sufficient separation through fractional vaporization alone.
- Reason: The addition of a selective solvent in extractive distillation helps modify the relative volatility of components, enhancing the separation of closely boiling substances.
- Improved Selectivity Requirements:
- Scenario: When there is a need for higher selectivity in separating specific components from a mixture.
- Reason: Extractive distillation allows for the strategic use of solvents that can selectively interact with certain components, leading to more precise separation.
- Enhanced Purity Requirements:
- Scenario: When the desired end product requires higher purity levels than what can be achieved through traditional distillation.
- Reason: Extractive distillation often results in higher-purity products, making it suitable for industries where stringent purity requirements are essential, such as pharmaceuticals or specialty chemicals.
- Minimal Volatility Differences:
- Scenario: When the components in the mixture have minimal differences in volatility, posing challenges for traditional distillation methods.
- Reason: Extractive distillation is effective in scenarios where the volatility differences between components are insufficient for efficient separation.
- Recycling Valuable Components:
- Scenario: When the goal is to recover and recycle valuable components from complex mixtures.
- Reason: Extractive distillation allows for the recycling of solvents and valuable components, contributing to sustainability and resource efficiency.
- Environmental Considerations:
- Scenario: When there are environmental concerns, and the process needs to be optimized for reduced energy consumption or emissions.
- Reason: Extractive distillation can be tailored to minimize environmental impact by optimizing energy usage and promoting efficiency in separation.
- Specific Industry Requirements:
- Scenario: When the industry has specific requirements that demand tailored separation processes.
- Reason: Extractive distillation is versatile and can be customized to meet the unique demands of various industries, including petrochemicals, pharmaceuticals, and renewable energy.
- Challenging Feedstock Composition:
- Scenario: When the feedstock composition is complex, and traditional distillation struggles to provide the desired product purity.
- Reason: Extractive distillation excels in handling complex mixtures, making it suitable for industries where the composition of the feedstock is diverse and challenging.
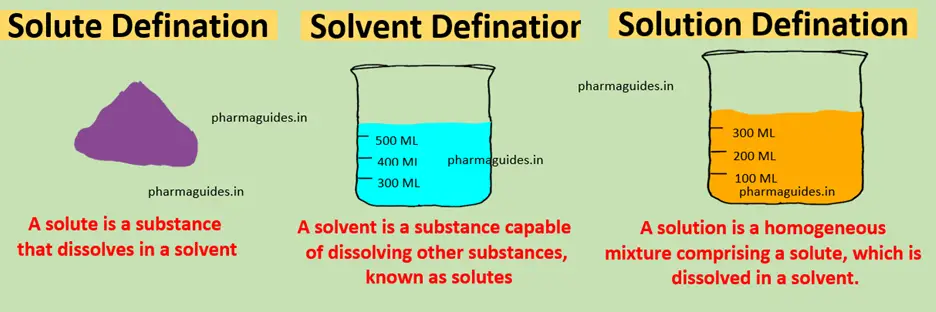
Solvent Used in Extractive Distillation
The choice of solvent in extractive distillation is a critical factor influencing the success of the process. Common solvents include polar and aprotic compounds like glycols, sulfolane, or even water. The selection process involves a comprehensive evaluation of compatibility with the mixture, boiling point differences, and the ease of recovery for reuse. The solvent serves not only as a separation agent but also as a strategic tool in achieving desired outcomes.
Solvent Selection Criteria in Extractive Distillation
Selecting the right solvent involves a nuanced consideration of several factors. The solvent should exhibit a distinct boiling point from the components of the mixture to ensure efficient separation. Additionally, it must possess favorable chemical and physical properties, such as stability, non-reactivity with the mixture, and ease of recovery for reuse. The careful selection of a solvent tailored to the specific mixture is crucial for the success of the extractive distillation process.
| Solvent Selection Criteria in Extractive Distillation | Considerations |
|---|---|
| Boiling Point Difference | The solvent should have a distinct boiling point from the components of the mixture to facilitate efficient separation during distillation. |
| Chemical Compatibility | The solvent must be chemically compatible with the mixture to avoid undesired reactions that could affect the separation process. |
| Stability and Inertness | The solvent should be stable under the operating conditions of the distillation process and inert towards the mixture components to prevent chemical changes. |
| Selectivity for Components | The solvent should exhibit selectivity for the target components, promoting preferential interaction for efficient separation. |
| Recoverability and Reusability | The solvent should be easily recoverable from the distillate and reusable in subsequent cycles to enhance the sustainability of the process. |
| Non-Corrosiveness | The solvent should be non-corrosive to the equipment and materials used in the distillation process to ensure longevity and reliability. |
| Economic Considerations | Considerations such as the cost, availability, and ease of procurement of the solvent play a crucial role in the economic feasibility of the process. |
| Environmental Impact | The environmental impact of the solvent, including toxicity and disposal considerations, should align with sustainability goals and regulatory requirements. |
| Viscosity and Density | The viscosity and density of the solvent should be compatible with the distillation process, ensuring smooth handling and efficient mass transfer. |
| Phase Equilibrium Behavior | The solvent should exhibit favorable phase equilibrium behavior with the mixture components to facilitate effective separation and prevent phase instability issues. |
Application of Extractive Distillation
Extractive distillation, with its unique ability to overcome the challenges posed by complex mixtures, finds application in various industries where precision separation and enhanced efficiency are paramount. Here are some notable applications:
Petrochemical Refineries
- Challenges: Petrochemical feedstocks often contain components with similar boiling points or form azeotropic mixtures, making separation challenging.
- Application: Extractive distillation is employed to separate key components such as benzene, toluene, and xylene (BTX) from complex hydrocarbon mixtures, improving product purity.
Pharmaceutical Manufacturing:
- Challenges: Pharmaceutical processes often involve the separation of closely boiling compounds or the removal of impurities.
- Application: Extractive distillation is utilized for purifying pharmaceutical intermediates, isolating specific compounds, and achieving high-purity products critical for pharmaceutical formulations.
Chemical Processing:
- Challenges: Chemical processes frequently encounter mixtures with components of similar volatility or azeotropic behavior.
- Application: Extractive distillation is applied to separate and purify chemicals in processes like the production of specialty chemicals, where traditional distillation methods may fall short.
Environmental Applications:
- Challenges: Environmental processes often involve the separation and recovery of pollutants or valuable components from complex waste streams.
- Application: Extractive distillation plays a role in environmental applications, such as separating volatile organic compounds (VOCs) from air emissions or recovering valuable chemicals from industrial waste streams.
Food and Beverage Industry:
- Challenges: Certain flavor compounds in food products may have similar boiling points, impacting the quality of the final product.
- Application: Extractive distillation is utilized to separate and concentrate specific flavor compounds, ensuring the production of high-quality food and beverage products.
Renewable Energy:
- Challenges: Biofuel production processes often involve the separation of ethanol-water mixtures, where traditional distillation faces limitations.
- Application: Extractive distillation is applied to improve the separation of ethanol from fermentation broths, contributing to more efficient biofuel production.
Natural Gas Processing:
- Challenges: Natural gas streams may contain impurities like sulfur compounds, requiring efficient separation methods.
- Application: Extractive distillation is employed to remove sulfur compounds from natural gas, contributing to the production of cleaner natural gas for various applications.
Fragrance and Perfume Industry:
- Challenges: Fragrance compounds may have similar boiling points, necessitating precise separation for high-quality products.
- Application: Extractive distillation is utilized to separate and purify fragrance components, ensuring the production of distinct and high-quality perfumes.
These applications underscore the versatility of extractive distillation in addressing a wide range of separation challenges across different industries. The method’s adaptability makes it a valuable tool for achieving targeted and efficient separations in complex mixtures, contributing to the advancement of various industrial processes.
Advantages and Disadvantages in Extractive Distillation
The advantages of extractive distillation include enhanced separation efficiency, the ability to tackle challenging mixtures, and improved product purity. However, the process demands a deep understanding of the mixture’s properties, and its energy-intensive nature may be a drawback in some cases. Despite the challenges, the strategic use of extractive distillation can lead to significant advancements in the efficiency and precision of separation processes.
| Advantages of Extractive Distillation | Disadvantages of Extractive Distillation |
|---|---|
| Enhanced Separation Efficiency: Extractive distillation improves separation efficiency, especially in challenging scenarios like azeotropic mixtures or those with similar boiling points. | Energy Intensive: The process can be energy-intensive due to the need for additional heat to facilitate vaporization and separation. |
| Strategic Solvent Use: The introduction of a solvent provides a strategic tool for tailoring the separation process to the specific mixture, enhancing the selectivity of separation. | Complex Process Design: Extractive distillation requires a deep understanding of the mixture’s properties and may involve complex process design, making it more challenging to implement. |
| Improved Product Purity: The method often results in higher-purity products, making it suitable for industries where stringent purity requirements are essential. | Solvent Selection Challenges: Choosing the right solvent is critical, and the selection process involves careful consideration of various factors, adding a layer of complexity to the process. |
| Recycling of Valuable Components: Extractive distillation allows for the recycling of valuable components from complex mixtures, contributing to sustainability and resource efficiency. | Equipment Compatibility: The use of a solvent may pose challenges related to the compatibility of materials with the solvent, potentially requiring specialized equipment. |
| Versatility in Applications: Extractive distillation finds applications across various industries, showcasing its versatility in addressing a wide range of separation challenges. | Initial Capital Costs: The setup for extractive distillation may involve higher initial capital costs compared to traditional distillation methods, impacting the economic feasibility of the process. |
This table provides a concise overview of the key advantages and disadvantages associated with extractive distillation, offering a quick reference for those considering its implementation in specific separation processes.
What do you mean by extractive distillation?
Extractive distillation is a specialized separation technique employed in chemical processes to enhance the efficiency of distillation. In this method, a third component, known as a solvent, is introduced into the system to alter the vapor-liquid equilibrium of the components in the mixture. The solvent selectively interacts with certain components, modifying their relative volatility and facilitating their separation. This technique is particularly useful in scenarios where traditional distillation struggles with challenges such as azeotropic mixtures or components with closely similar boiling points.
What is the difference between extractive and azeotropic distillation?
While both extractive and azeotropic distillation involve the addition of a third component to aid in separation, they differ in their primary objectives. Extractive distillation focuses on improving the separation of components in a mixture by modifying their relative volatility through the addition of a selective solvent. Azeotropic distillation, on the other hand, aims to break or enhance the formation of azeotropes, which are constant-boiling mixtures, often by adding a compound that forms a new azeotrope with the mixture components.
What is extractive and reactive distillation?
Extractive distillation and reactive distillation are both advanced distillation techniques. Extractive distillation involves the addition of a solvent to improve separation, as discussed earlier. Reactive distillation, on the other hand, integrates a chemical reaction into the distillation process. The chemical reaction aids in the separation of components, and simultaneously, the distillation process helps drive the reaction towards completion. While both techniques aim to enhance separation, they achieve it through different mechanisms: solvent addition in extractive distillation and simultaneous reaction and distillation in reactive distillation.
What is the difference between extractive distillation and fractional distillation?
The primary difference between extractive distillation and fractional distillation lies in the involvement of a third component, the solvent. In fractional distillation, the separation is based on the differences in boiling points of the components in the mixture, and no additional substance is introduced. Extractive distillation, however, utilizes a solvent strategically to modify the vapor-liquid equilibrium and enhance the separation efficiency. Fractional distillation is a more traditional and straightforward method, while extractive distillation adds a layer of complexity to achieve more precise separations.
Why extractive distillation?
Extractive kind of distillation is employed for several reasons. It is particularly valuable when dealing with challenging mixtures, such as azeotropic ones or those with components having similar boiling points. The strategic addition of a solvent allows for more precise separation, improved efficiency, and higher product purity. Extractive type of distillation becomes a strategic choice in industries where traditional distillation methods fall short, providing a tailored approach to separation challenges.
Difference Between Azeotropic and Extractive Distillation
What is extractive process?
An extractive process, in a broader sense, refers to any technique or method where a specific substance is selectively extracted or separated from a mixture. Extractive processes can be found in various industries, from chemistry to food processing. In the context of extractive distillation, it specifically involves the addition of a solvent to modify the separation characteristics of components in a mixture, emphasizing precision and efficiency in the separation process. The extractive process, whether in distillation or other applications, is characterized by its selectivity and strategic use of additional agents for improved separation outcomes.
Conclusion
Extractive distillation stands out as a sophisticated and valuable player in the realm of separation processes. Its ability to navigate the intricacies of challenging mixtures, coupled with strategic solvent selection, positions it as an indispensable tool in industries requiring precision and efficiency in their separation processes. While not a universal solution, extractive kind of distillation pushes the boundaries of what is achievable in the world of chemical processing, offering a tailored approach to the complexities of separation.
What is extractive distillation in distillation



1 COMMENTS
Comments are closed.