Article Contents
maximum boiling azeotrope
Unlocking the Potential of Maximum Boiling Azeotropes: A Fascinating Journey into the World of Liquid Mixtures
In the realm of liquid mixtures, azeotropes hold a special place due to their intriguing behavior. Among them, maximum boiling azeotropes stand out as captivating phenomena with unique properties and applications. These peculiar mixtures, also known as positive azeotropes, present fascinating challenges and opportunities for scientists and engineers. This article delves into the concept of maximum boiling azeotropes, explores their characteristics, and highlights their significance in various fields, the system that forms maximum boiling azeotrope is.
Understanding Azeotropes
Before delving into maximum boiling azeotropes, it is crucial to understand the fundamental concept of azeotropy. An azeotrope is a mixture of two or more substances that exhibits a constant boiling point, meaning that the liquid mixture vaporizes without a change in composition. Azeotropes can be categorized into three types: minimum boiling, maximum boiling, and ternary azeotropes.

Maximum Boiling Azeotropes: A Peculiar Alliance
Maximum type of boiling azeotropes are unique among azeotropes due to their distinct behavior. Unlike minimum boiling azeotropes, which have boiling points lower than those of their constituents, maximum boiling azeotropes have boiling points higher than the individual components. This counterintuitive characteristic arises from the synergistic interactions between the constituents, leading to a change in the vapor-liquid equilibrium ,the mixture that forms maximum boiling azeotrope is.
Composition and Behavior
The composition of a maximum type of boiling azeotrope is critical to understanding its behavior. The mixture ratio of the components determines the boiling point of the azeotrope. Interestingly, when the mixture is distilled, the vapor produced will have the same composition as the original liquid, regardless of the initial composition. This behavior makes it challenging to separate the components by conventional distillation techniques, a binary liquid mixture that forms maximum boiling azeotrope at a specific composition.
Applications in Distillation and Separation Processes
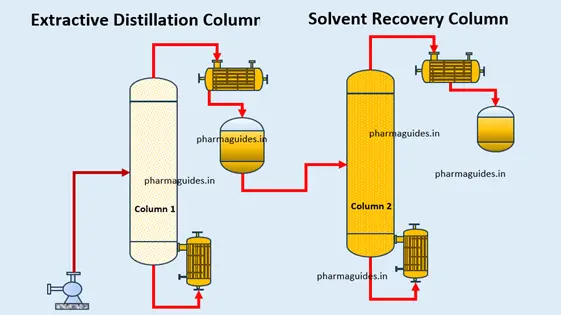
While the behavior of maximum boiling azeotropes poses challenges in separation processes, it also presents opportunities for innovation. Advanced distillation techniques such as extractive distillation and azeotropic distillation have been developed to overcome the limitations imposed by azeotropes. These methods utilize additional components called entrainers to modify the azeotropic behavior, enabling efficient separation of the constituents.
In the chemical and pharmaceutical industries, maximum boiling azeotropes play a crucial role in the purification and separation of various compounds. They are employed in the production of ethanol, benzene, toluene, and cyclohexane, among others. By carefully selecting entrainers and optimizing process parameters, scientists and engineers can achieve high-purity separations that were once considered challenging or impossible, maximum boiling azeotrope examples.
Beyond Separation: Maximum kind Boiling Azeotropes in Other Applications
Apart from their significance in separation processes, maximum kind of boiling azeotropes find applications in other fields as well. Their unique properties are utilized in heat pumps and refrigeration systems, where they serve as working fluids. The stability of their boiling point simplifies the design and operation of such systems.
Moreover, maximum kind of boiling azeotropes have found applications in the field of green chemistry. Their ability to facilitate energy-efficient separations and reduce the use of hazardous solvents makes them attractive alternatives for environmentally conscious processes.
Future Directions and Research Challenges
Although maximum boiling azeotropes have been extensively studied, several challenges and research opportunities lie ahead. Developing novel entrainers and optimizing their use in separation processes is an active area of research. Furthermore, exploring the behavior of ternary azeotropes and expanding our understanding of complex liquid mixtures will unlock new possibilities for efficient separation techniques.
What are maximum boiling azeotropes with examples?

Maximum boiling azeotropes are mixtures that have a boiling point higher than the boiling points of their individual components. Here are a few examples:
a) Nitric acid-water: The maximum boiling azeotrope of nitric acid and water contains approximately 68% nitric acid and has a boiling point of around 121 degrees Celsius. This azeotrope is important in the production of concentrated nitric acid , a binary liquid mixture that forms maximum boiling azeotrope at a specific composition is.
b) Hydrochloric acid-water: The maximum boiling azeotrope of hydrochloric acid and water consists of approximately 20.2% hydrochloric acid and has a boiling point of about 110 degrees Celsius. This azeotrope finds applications in various chemical processes.
c) Sulfuric acid-water: The maximum boiling azeotrope of sulfuric acid and water is composed of approximately 98.3% sulfuric acid and has a boiling point of around 338 degrees Celsius. This azeotrope is utilized in the production of concentrated sulfuric acid.
What is the difference between minimum and maximum boiling azeotropes? The difference between minimum boiling azeotropes and maximum boiling azeotropes lies in their boiling point behavior.
Minimum boiling azeotropes: These mixtures have a boiling point lower than the boiling points of their individual components. When a minimum boiling azeotrope is distilled, the vapor produced has a different composition than the original liquid mixture. This allows for separation of the components through distillation.
Maximum boiling azeotropes: These mixtures have a boiling point higher than the boiling points of their individual components. When a maximum boiling azeotrope is distilled, the vapor produced has the same composition as the original liquid mixture. This makes the separation of components through conventional distillation challenging.
What is the system for maximum boiling azeotropes?
The system for maximum kind of boiling azeotropes typically involves two or more components that form an azeotropic mixture with a boiling point higher than any of the individual components. These components interact in a way that leads to the formation of the azeotrope with a maximum boiling point. The behavior of maximum kind of boiling azeotropes is determined by the specific combination of substances and their concentrations.
Diagram of Fractional distillation
What is a high boiling azeotropic mixture?
A high boiling type of azeotropic mixture refers to an azeotrope that has a boiling point significantly higher than the boiling points of its constituents. The term “high boiling” indicates that the boiling point of the azeotrope is elevated compared to the boiling points of the individual components. High boiling azeotropic mixtures are often encountered in industrial processes where the separation of components with similar boiling points is challenging. Advanced techniques such as extractive distillation or azeotropic distillation may be employed to overcome these challenges and achieve efficient separation.
Conclusion
c offer a captivating journey into the world of liquid mixtures. Their peculiar behavior, characterized by boiling points higher than their constituents, presents both challenges and opportunities in separation processes and other applications. By harnessing the synergistic effects of azeotropes and developing innovative techniques, scientists and engineers continue to unravel the potential of these intriguing mixtures, paving the way for advancements in various fields.
Read Also,
maximum and minimum boiling azeotropes examples





1 COMMENTS
Comments are closed.