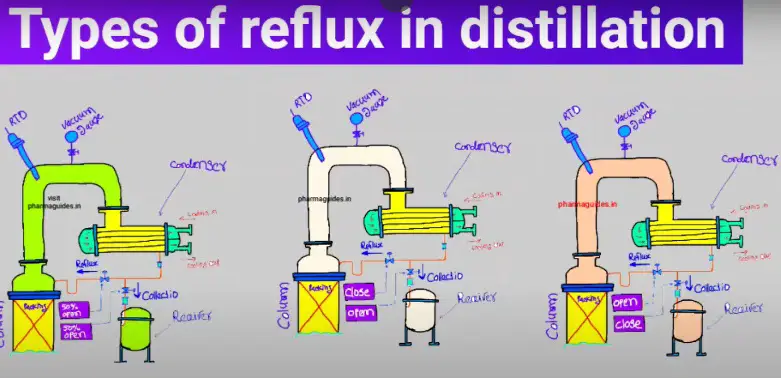
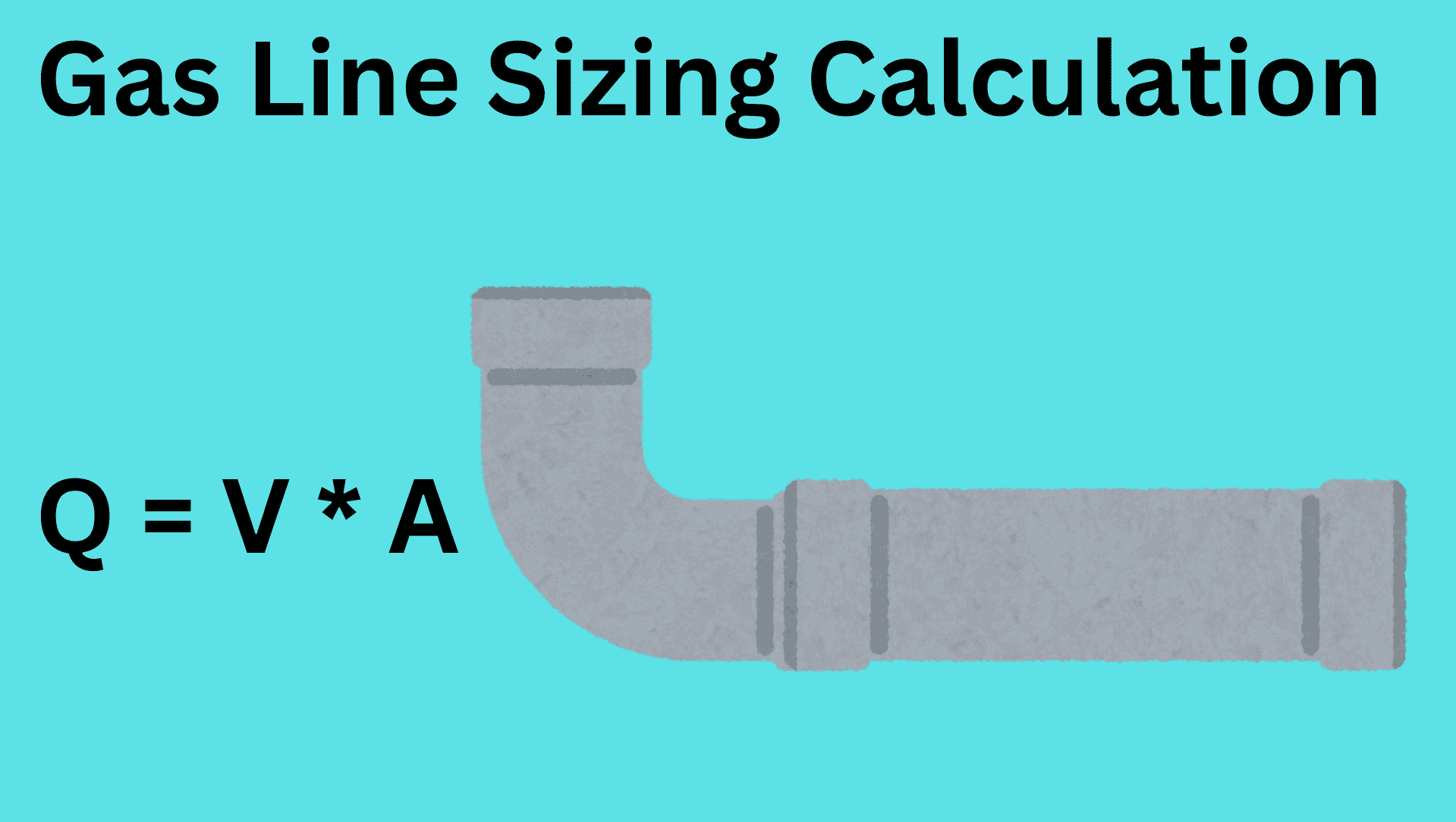
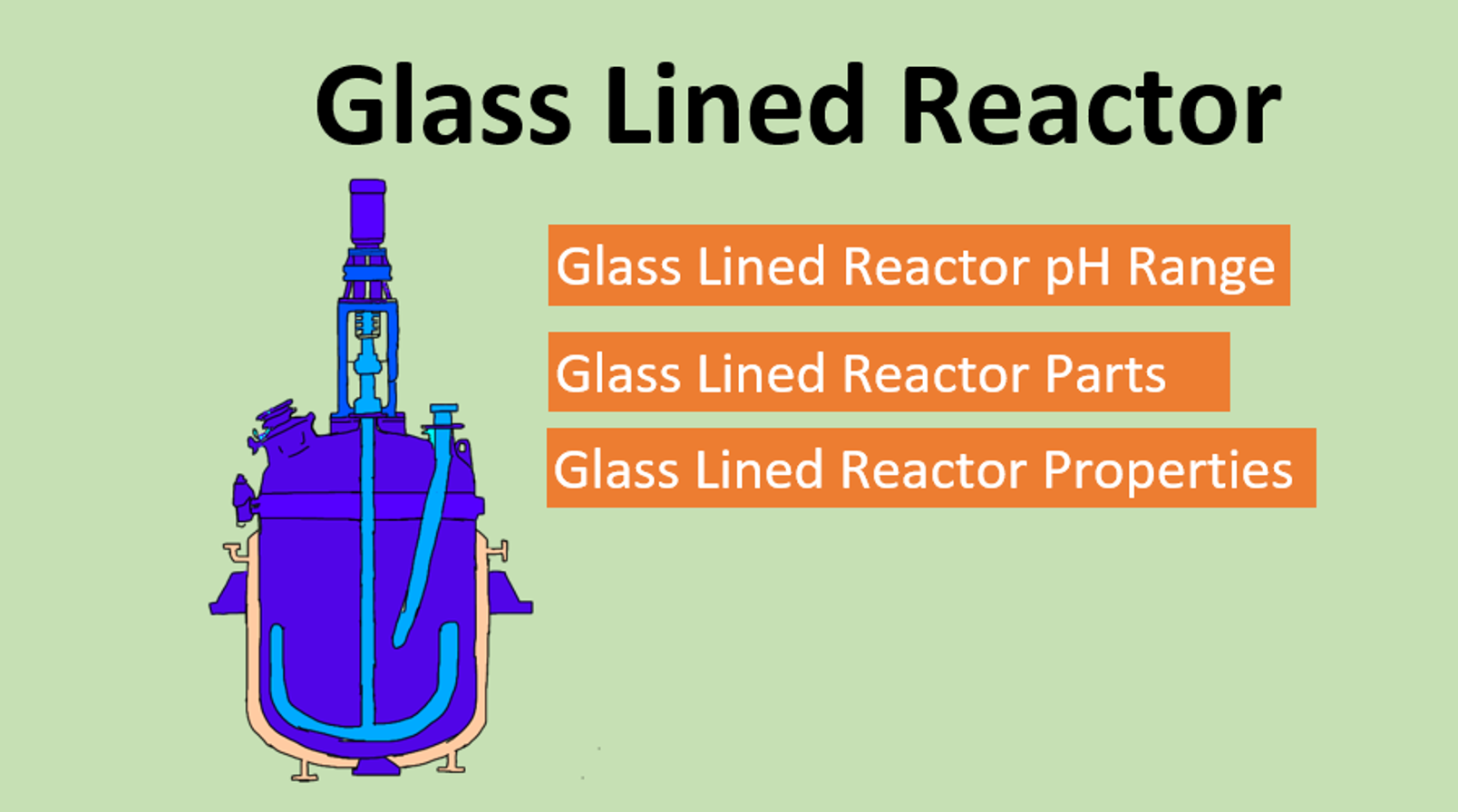
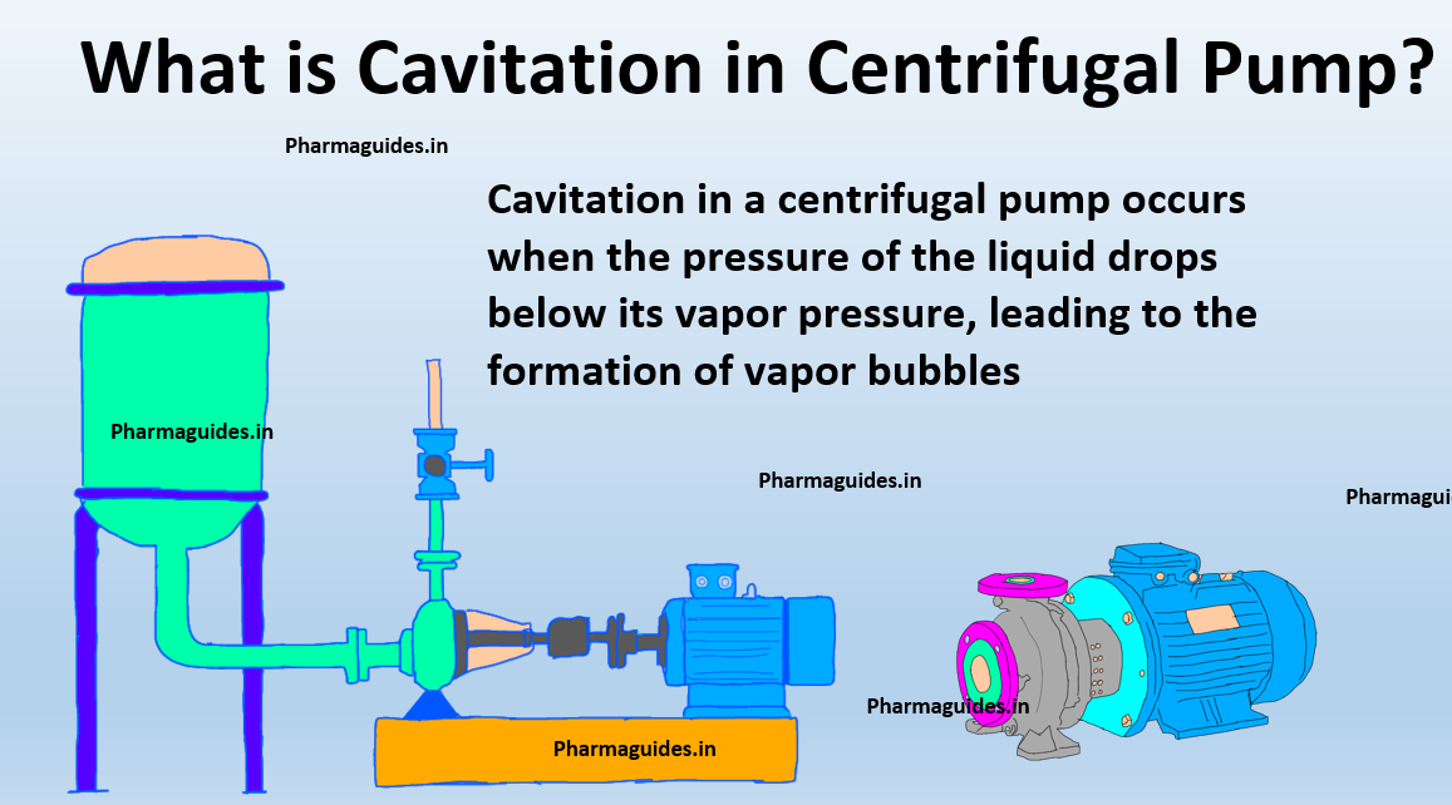
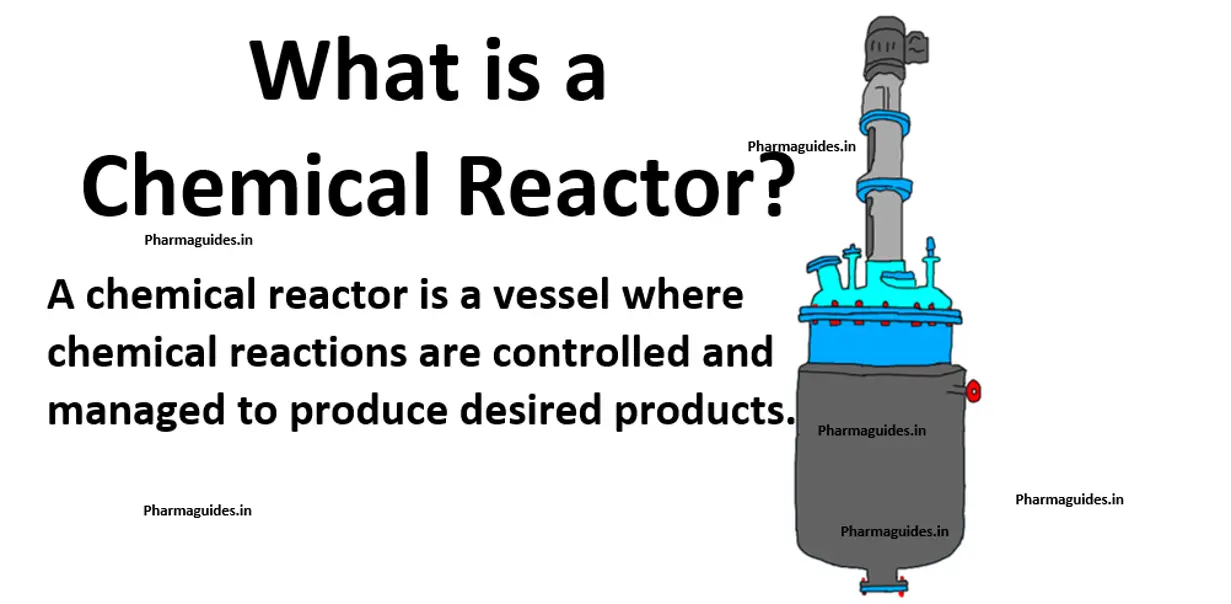
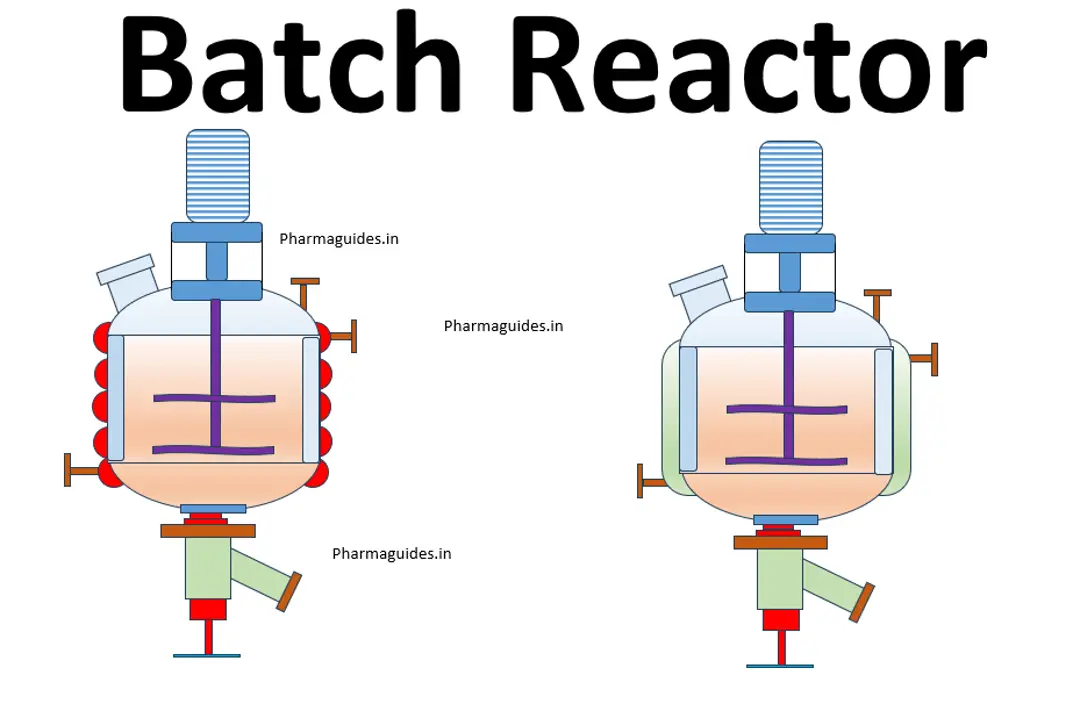
chemical reactions and equations class 10 notes with pdf free
Chemical reactions and equations class 10 notes As an AI language model, I don’t have access to specific documents or files, including CBSE class 10 notes. However, I can provide you with a brief overview of chemical reactions and equations,…