raoult law class 12
Raoult’s law is a fundamental concept in chemistry, specifically in the field of solutions. It describes the behavior of ideal solutions, which are solutions that obey certain assumptions. Raoult’s law states that the partial pressure of each component of an ideal solution is directly proportional to its mole fraction in the solution.
Here are some important points to understand about raoult law class 12 :
- Ideal Solution: raoult law class 12 applies to ideal solutions, which are solutions that exhibit no deviation from ideal behavior. In an ideal solution, the intermolecular interactions between different components are assumed to be similar to the interactions between like molecules. This means that the solute-solute, solvent-solvent, and solute-solvent interactions are all similar in nature.
- Partial Pressure: raoult law class 12 relates the partial pressure of a component in the vapor phase to its mole fraction in the liquid phase. The partial pressure of a component is the pressure exerted by that component when it is in the vapor phase above the solution.
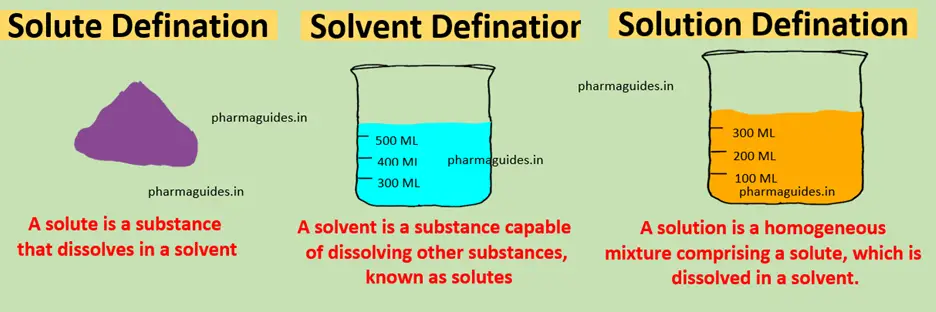
- Mole Fraction: The mole fraction of a component in a solution is the ratio of the number of moles of that component to the total number of moles of all components present in the solution. It is denoted by the symbol “x” followed by the component’s chemical formula.
- Mathematical Formulation: Raoult’s law can be expressed mathematically as follows: For a component i in an ideal solution:
Pi = xi * P
where Pi is the partial pressure of component i, xi is the mole fraction of component i, and P is the total pressure of the solution. The sum of the mole fractions of all components in the solution is equal to 1:
x1 + x2 + … + xn = 1 - Ideal Behavior: Raoult’s law assumes that the vapor phase of an ideal solution behaves ideally, meaning that the vapor pressure of each component is directly proportional to its mole fraction in the liquid phase. This implies that the components evaporate independently of each other and do not interact in the vapor phase.
- Non-Ideal Solutions: Real solutions often deviate from ideal behavior due to various factors such as intermolecular interactions, association or dissociation of solute molecules, or significant differences in the sizes of solute and solvent molecules. In such cases, Raoult’s law does not hold true, and the observed behavior may deviate from the predictions of the law.
- Applications: Raoult’s law is particularly useful in studying the behavior of volatile liquid mixtures, such as solutions involving two or more volatile liquids. It helps in determining the vapor pressures, boiling points, and composition of such mixtures.
It’s important to note that while raoult law class 12 is a useful approximation for certain solutions, it does not account for deviations from ideal behavior. To describe non-ideal solutions, other thermodynamic models, such as Henry’s law, activity coefficients, or equations of state, are employed.
People Also Aksed.
Here are ten questions related toraoult law class 12 along with their answers:
Question: What is Raoult’s law?
Answer: Raoult’s law states that the partial pressure of each component of an ideal solution is directly proportional to its mole fraction in the solution.
Question: Under what conditions does Raoult’s law hold true?
Answer: Raoult’s law holds true for ideal solutions, where the solute-solvent interactions are similar to solvent-solvent interactions.
Question: What is the significance of Raoult’s law in studying volatile liquid mixtures?
Answer: Raoult’s law helps determine the vapor pressures, boiling points, and composition of volatile liquid mixtures.
Question: What happens to the vapor pressure of a solvent in the presence of a non-volatile solute?
Answer: The vapor pressure of the solvent decreases in the presence of a non-volatile solute according to Raoult’s law.
Question: How does Raoult’s law relate to colligative properties?
Answer: Raoult’s law provides a basis for understanding the vapor pressure lowering effect, which is one of the colligative properties of solutions.
Question: Can Raoult’s law be applied to non-ideal solutions?
Answer: No, Raoult’s law is not applicable to non-ideal solutions where deviations from ideal behavior occur.
Question: What are some factors that can cause deviations from Raoult’s law?
Answer: Factors such as intermolecular interactions, association or dissociation of solute molecules, and differences in the sizes of solute and solvent molecules can cause deviations from raoult law class 12.

Question: How does Raoult’s law help in constructing phase diagrams?
Answer: Raoult’s law is used to determine the vapor pressures of components at various compositions, aiding in the interpretation of phase diagrams.
Question: Is Raoult’s law more applicable to dilute or concentrated solutions?
Answer: Raoult’s law is more applicable to dilute solutions where the solute concentration is relatively low compared to the solvent.
What does Raoult’s Law state?
Raoult’s Law states that the partial pressure of each component in an ideal solution is directly proportional to its mole fraction in the solution. It describes the behavior of ideal solutions where the solute-solvent interactions are similar to solvent-solvent interactions.
What is Raoult’s Law Class 12 Doubtnut?
“Doubtnut” is an online learning platform that provides educational resources for various subjects, including Class 12 Chemistry. raoult law class 12 Doubtnut likely refers to specific lessons, explanations, or practice questions related to Raoult’s Law offered by the Doubtnut platform for Class 12 students studying Chemistry.
What is Raoult’s law in India?
Raoult’s Law is a fundamental concept in chemistry and is not specific to any particular country, including India. It is a universally accepted principle that describes the behavior of ideal solutions. The application and understanding of Raoult’s Law in India would be the same as in any other country, what is raoult law class 12.
Question: What are some limitations of Raoult’s law?
Answer: Raoult’s law assumes ideal behavior, which may not hold true for real solutions. It does not account for deviations from ideal behavior, such as non-ideal interactions between solute and solvent molecules.
diagram of fractional distillation
Related searches
positive and negative deviation from raoult’s law examples
difference between positive and negative deviation from
what is raoult law class 12