distillation definition- why is the process of fractional distillation used in refineries? 2.0
Article Contents
distillation definition
What is distillation ?
Simple Distillation
why is the process of fractional distillation used in refineries?
simple definition of fractional distillation
Fractional distillation definition is a separation process used to separate a mixture of liquids with different boiling points. It involves heating the mixture in a column or distillation tower, and as the temperature increases, the components with lower boiling points vaporize first. The vapor then rises through the column, where it condenses and re-vaporizes multiple times due to temperature gradients. This repeated vaporization and condensation, along with the column’s packing or trays, allows for better separation of the components based on their boiling points. The purified components are collected at different levels of the column, resulting in the separation of the original mixture into its individual constituents.
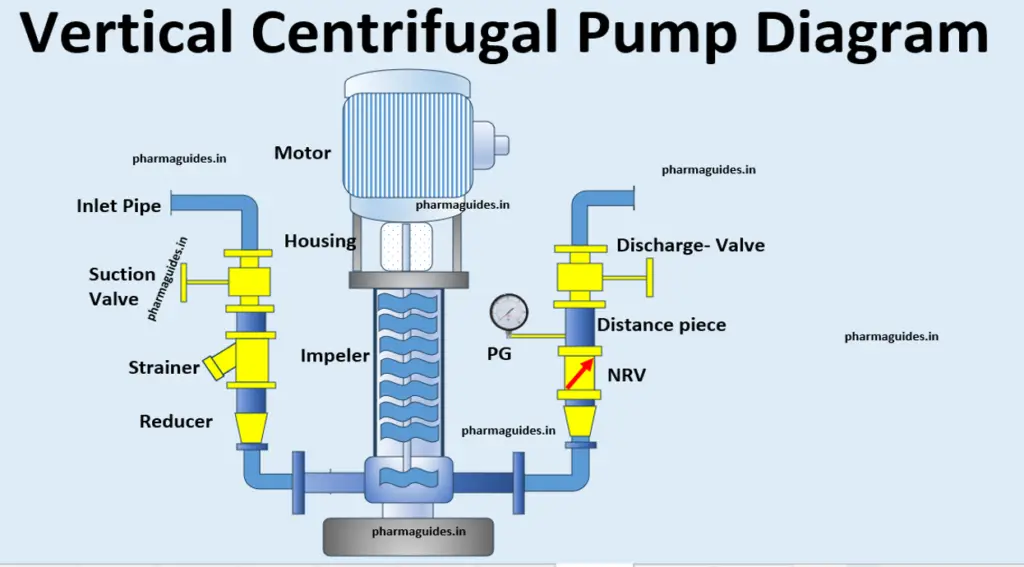
Fractional distillation definition contains ,reboiler ,fractional column, condenser ,pumps. what is distillation ?
Problems in Fractional distillation column
- Flooding –
- Pressure drop
- Weeping
- Dumping
- coning
Azeotropic Distillation
Azeotrop will form when relative volatility is one.In Azeotropic distillation added third component or solvent is called as entrainer. After addition of solvent in azeotropic mixture ,solvent brake the azeotrope of mixture and form new azeotrope of low boiling mixture .
Steam Distillation
Destructive Distillation
Vacuum distillation
High Pressure Distillation
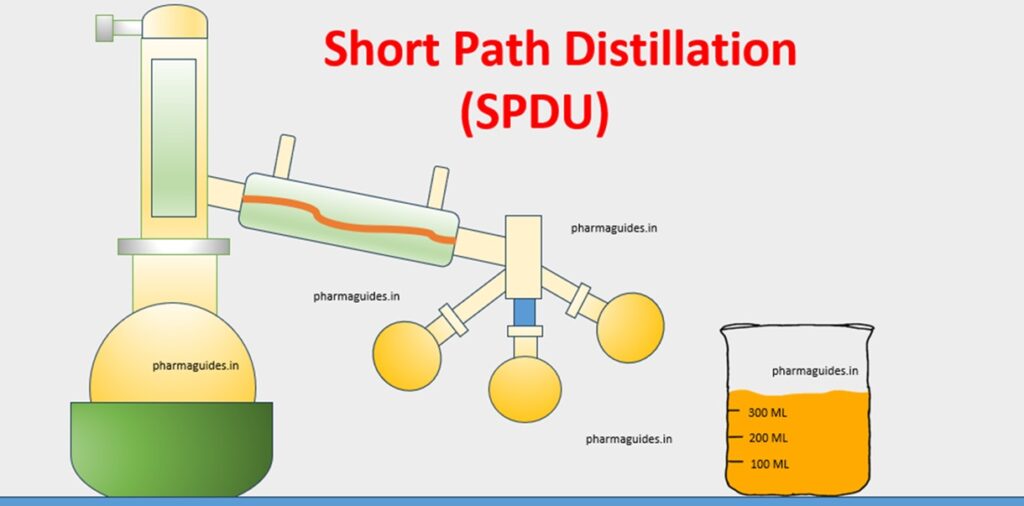
Short path Distillation
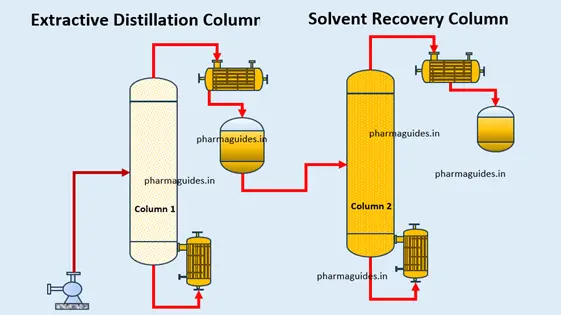
Extractive Distillation
Distillation Process
In general ,we feed the crude mixture in distillation column,reboiler or evaporator ,evaporate that mixture .After evaporation of mixture in evaporator,vapor comes to stripping section.In striping section low volatile mixture get condensed by liquid coming down(Re-flux & feed) in distillation column.
After Stripping section liquid will go to rectification section.In rectification section low volatile components from reflux liquid will go with vapor. After rectification finally vapors go to condenser ,condenser condense the vapor and we will get the condensate .
Above process is process of fractional distillation ,normally each type of distillation have some difference in there process.
Factor affecting distillations
- Relative volatility of mixture →You need to calculate proper relative volatility .
- Solubility
- Maximum possible concentration
- Activity coefficient
- Surface Area
- Water purification – Best way to purify water is boil them or do distillation to get pure quality of water. Pure or distilled water is required for drinking purpose . High grade distilled water required for cooling engine, HPLC ,GC.
- Solvent recovery – this is most widely used in pharmaceutical, chemical industry.
- Crude oil distillation – In petrochemical industry distillation is used to separate petroleum products like petrol, diesel, kerosene, methane etc. In petrochemical industry or in oil refinery petrochemical products separate from crude to use in different types of application.
- Distillery industries – for production of alcohol.
- Food industries
- Pharmaceutical industries
- Gas industries
- Oil industries
- Colors industries
People also ask
Question: What is fractional distillation in chemistry class 9?
Answer: Fractional distillation is a separation technique used in chemistry class 9 to separate a mixture of liquids with different boiling points. It involves heating the mixture, and as the temperature rises, the components with lower boiling points vaporize first. The vapors then rise through a fractionating column, where they condense and re-vaporize multiple times. This process allows for better separation based on boiling points, and the purified components are collected at different levels of the column.
Question: What is fractional distillation Class 9 example?
Answer: An example of fractional distillation in Class 9 could be the separation of a mixture of ethanol (boiling point around 78°C) and water (boiling point around 100°C). By heating the mixture, the ethanol with the lower boiling point will vaporize first and rise through the fractionating column, while the water remains in the liquid state. The condensation and re-vaporization in the column result in the separation of ethanol from the water, allowing the collection of the purified ethanol.
Question: What is fractional distillation in simple words?
Answer: Fractional distillation, simply put, is a method to separate a mixture of liquids with different boiling points. By heating the mixture and using a fractionating column, the components with lower boiling points vaporize first and then condense and re-vaporize multiple times. This process helps to achieve better separation of the components, and the purified substances are collected at different levels.
Question: What is the process of fractional distillation of air Class 9?
Answer: In Class 9, the fractional distillation of air is a process used to separate the various components of air, primarily nitrogen, oxygen, and argon. Air is compressed and cooled to very low temperatures, around -196°C, turning it into a liquid. The liquid air is then slowly heated and allowed to vaporize in a fractionating column. As the temperature rises, the components with lower boiling points, such as nitrogen and argon, vaporize first and are collected at different levels of the column. Oxygen, with a slightly higher boiling point, remains in the liquid state and can be collected separately. This process enables the isolation of pure nitrogen, oxygen, and argon from the air ,definition of fractional distillation.